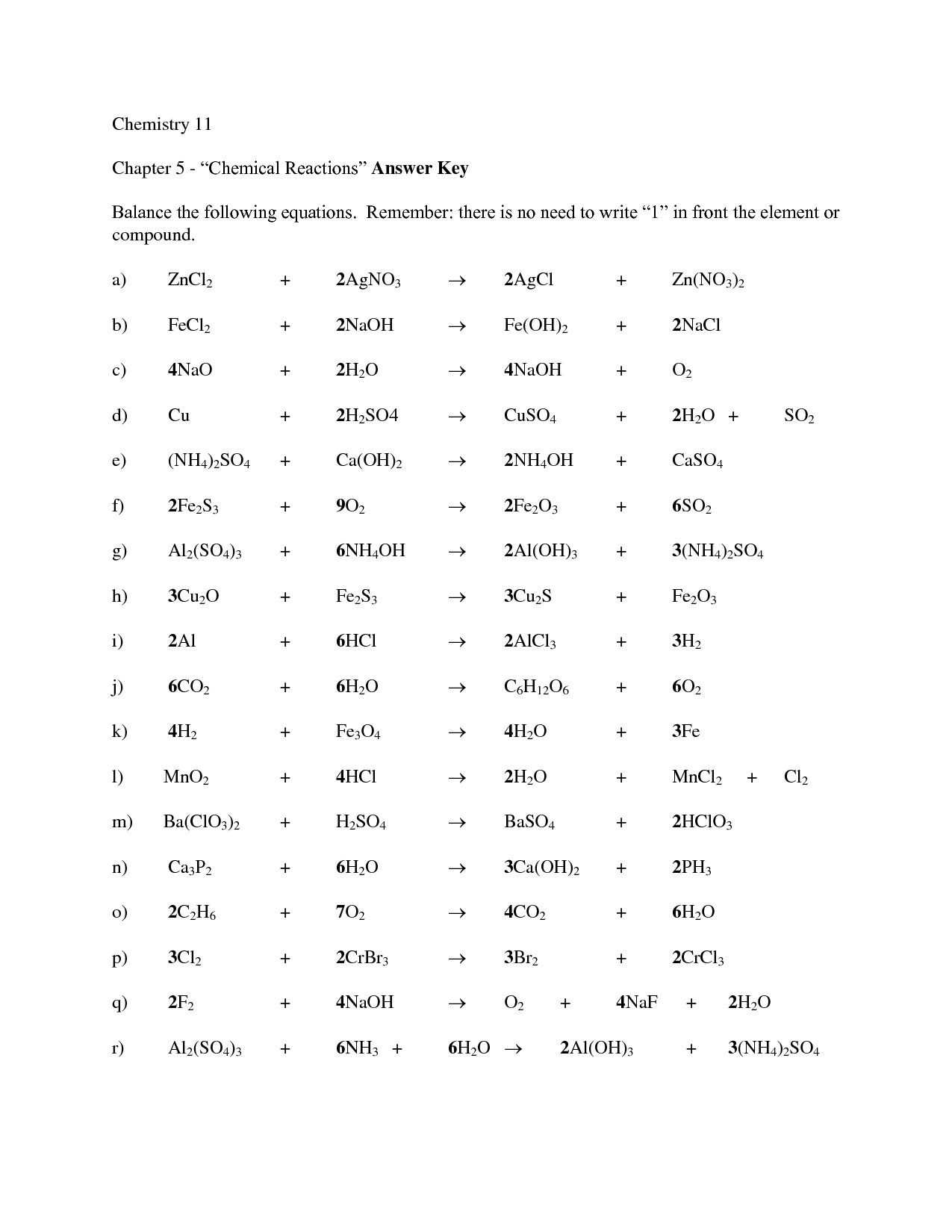
Writing And Balancing Chemical Equations Worksheet
A balanced chemical equation gives the number and type of atoms participating in a reaction, the reactants, products, and direction of the reaction. Balancing an unbalanced equation is mostly a matter of making certain mass and charge are balanced on the reactants and products side of the reaction arrow.

Balancing Chemical Equations Worksheet 1 Answers —
Step 1 The unbalanced equation must be obtained from the chemical formulae of the reactants and the products (if it is not already provided). The chemical formula of propane is C 3 H 8. It burns with oxygen (O 2) to form carbon dioxide (CO 2) and water (H 2 O) The unbalanced chemical equation can be written as C3H8 + O2 → CO2 + H2O Step 2

Chemistry Balancing Equations Practice Worksheet Answer Key / Balancing Chemical Equations
Balancing chemical equations 1 Google Classroom Balance the following chemical equation: Mg (OH) 2 + HCl → MgCl 2 + H 2 O Note: All reactants and products require a coefficient of at least one. Stuck? Review related articles/videos or use a hint. Report a problem

Balancing Equation Worksheet With Answers
PROBLEM 5.1.1. 4. Write a balanced equation describing each of the following chemical reactions. Solid potassium chlorate, KClO 3, decomposes to form solid potassium chloride and diatomic oxygen gas. Solid aluminum metal reacts with solid diatomic iodine to form solid Al 2 I 6.

Balancing Equation Worksheet With Answers
The ultimate goal for balancing chemical equations is to make both sides of the reaction, the reactants and the products, equal in the number of atoms per element. This stems from the universal law of the conservation of mass, which states that matter can neither be created nor destroyed.

Balancing Equations Questions and Answers
49 Balancing Chemical Equations Worksheets [with Answers] Do you find balancing the chemical equation a daunting task? If yes, then you may also get confused playing with the molecules and atoms. You have to balance the chemical equation no matter what, as per the Law of Conservation of Matter, but many students find it difficult to balance it.

balancing equations worksheet answers Chemical Substances Iron
Balancing Chemical Equations. A balanced equation is a chemical equation in which mass is conserved and there are equal numbers of atoms of each element on both sides of the equation. We can write a chemical equation for the reaction of carbon with hydrogen gas to form methane (CH4): C(s) 2Catoms + H2(g) 2Hatoms → CH4(g) 1 Catom,4H atoms.
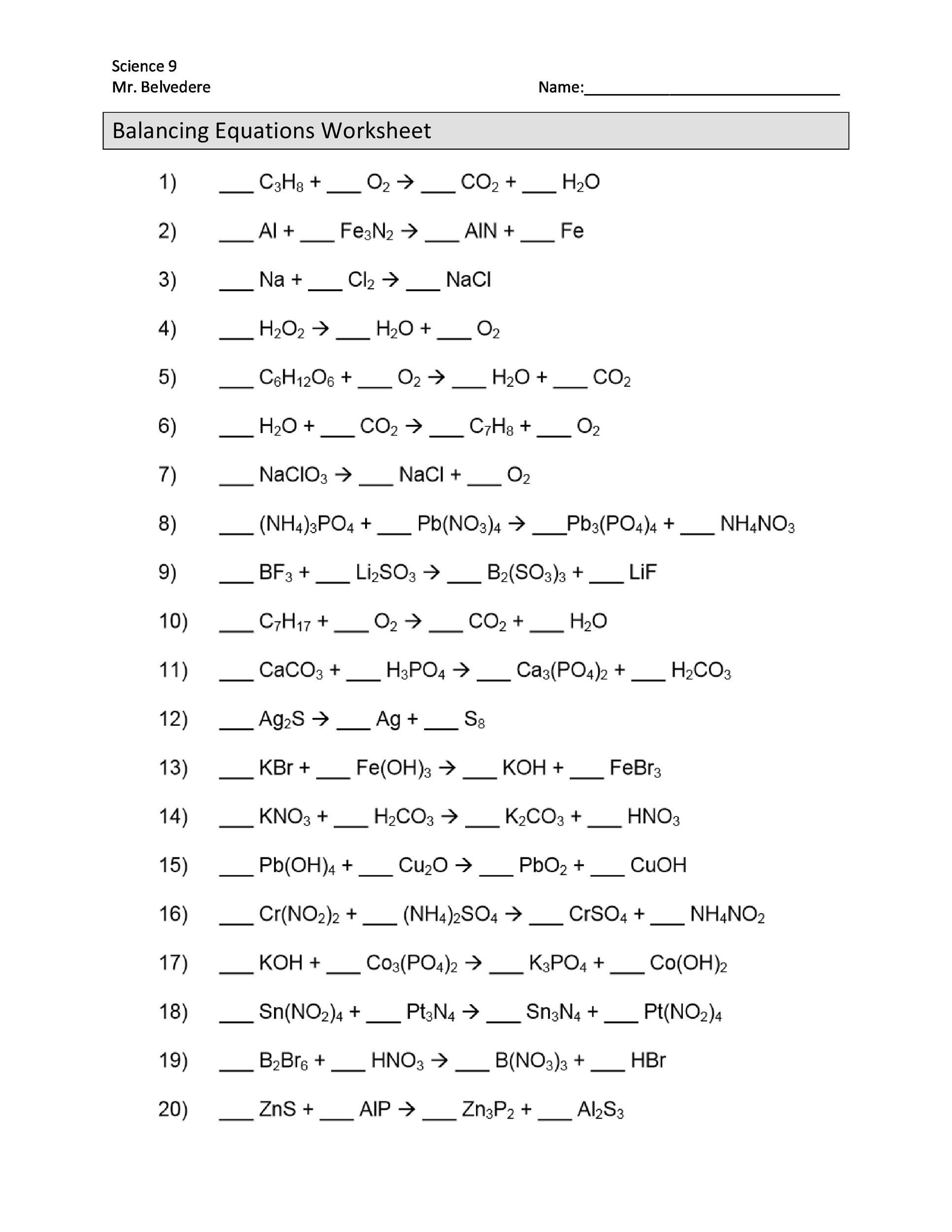
49 Balancing Chemical Equations Worksheets [with Answers]
Identify Type of Reaction. Single Displacement. Balance ions not atoms (Video 3.3a) Double and Single Displacement. Balance ions not atoms (Video 3.3b) Strategy is pick an ion, do its counterion, then do the counterion's counterion, and repeat, until all have been balanced (watch video). Combustion.

49 Balancing Chemical Equations Worksheets [with Answers]
It takes practice to be able to write balanced equations. There are essentially three steps to the process: 1. Write the unbalanced equation. Chemical formulas of reactants are listed on the left side of the equation. Products are listed on the right side of the equation.

Chemistry Balancing Chemical Equations Practice Worksheet Answer Key › Athens Mutual Student Corner
The chemical equation described in section 4.1 is balanced, meaning that equal numbers of atoms for each element involved in the reaction are represented on the reactant and product sides. This is a requirement the equation must satisfy to be consistent with the law of conservation of matter. It may be confirmed by simply summing the numbers of.
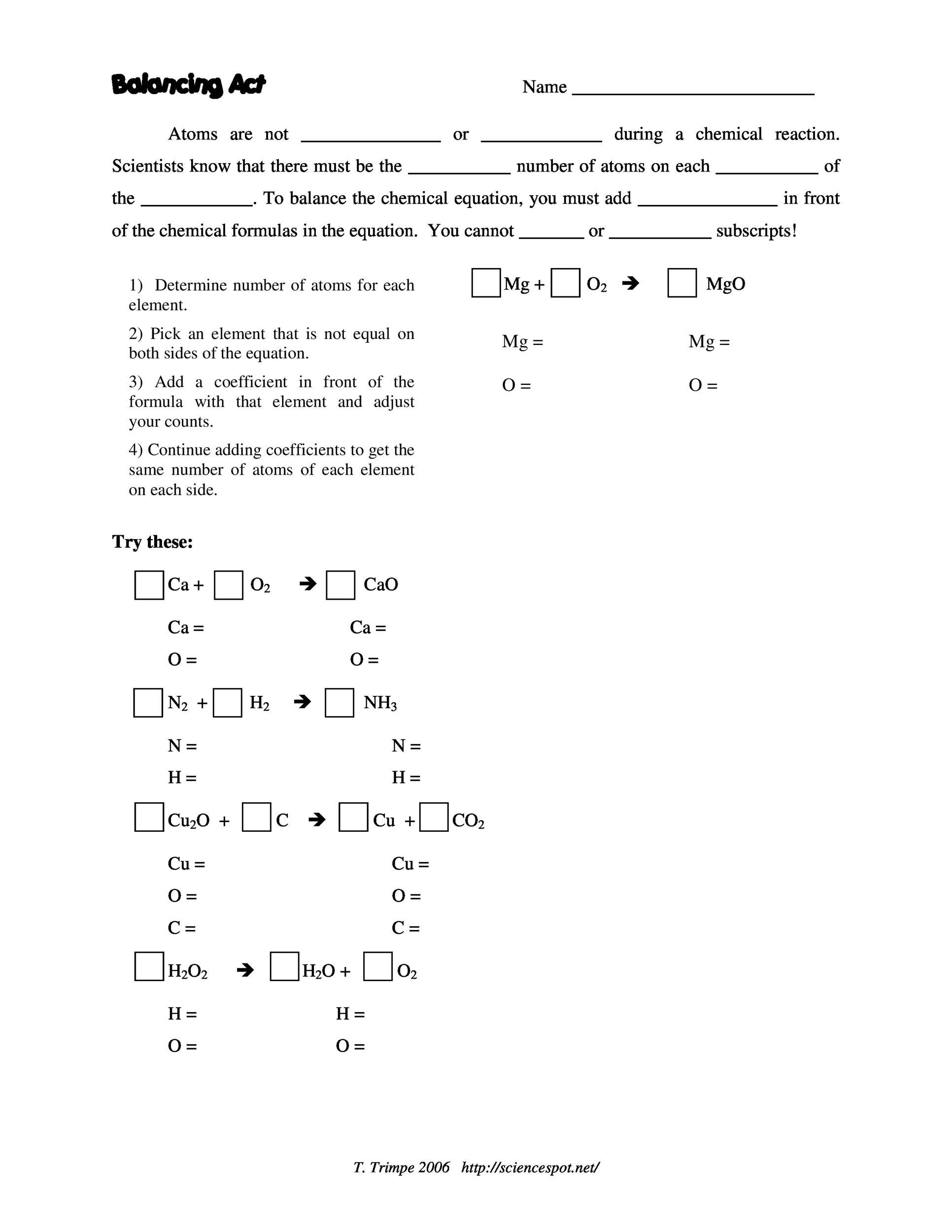
49 Balancing Chemical Equations Worksheets [with Answers]
1. Balance each of the following equations. Balancing Equations: Answers to Practice Problems Balanced equations. (Coefficients equal to one (1) do not need to be shown in your answers). 2 Fe+ 3 Cl2 − −→ 2 FeCl3 4 Fe+ 3 O2 − − → 2 Fe2O3 2 FeBr + − −→ + 3 3 H2SO4 1 Fe2(SO4) 3 (d) 1 C4H6O3 + 1 H2O − −→ 2 C2H4O2 (e) 1 C2H4 + 3 O2 − −→ 2 CO2 + 2 H2O
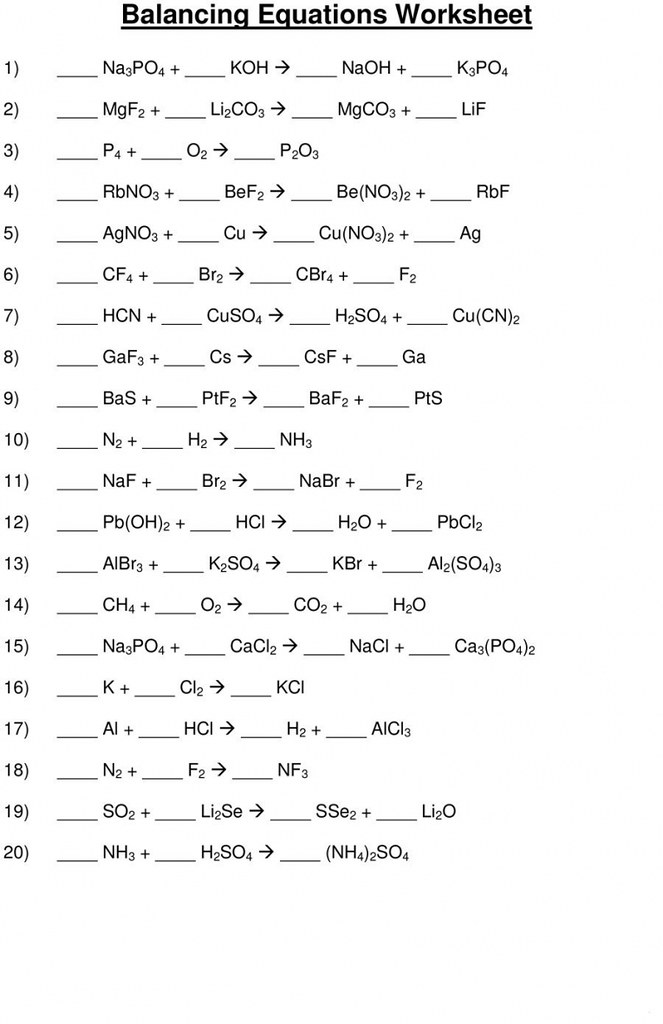
Balancing Chemical Equations Worksheet Balancing Chemical … Flickr
These coefficients yield equal numbers of both H and O atoms on the reactant and product sides, and the balanced equation is, therefore: H2O H2 +O2 (balanced) (balanced) H 2 O H 2 + O 2. Example 4.1.1 4.1. 1: Balancing Chemical Equations. Write a balanced equation for the reaction of molecular nitrogen (N 2) and oxygen (O 2) to form dinitrogen.

Identifying Reaction Types And Balancing Equations Worksheet
Balancing chemical equations is a basic skill in chemistry and testing yourself helps retain important information. This collection of ten chemistry test questions will give you practice in how to balance chemical reactions . Question 1 Balance the following equation: __ SnO 2 + __ H 2 → __ Sn + __ H 2 O Question 2 Balance the following equation:

Answer Key Balancing Chemical Equations Practice Worksheet With Answers kidsworksheetfun
How do you know if a chemical equation is balanced? What can you change to balance an equation? Play a game to test your ideas!

[Grade 9 Physical Science Chemical Reactions] Balancing equations We were given this worksheet
Instructions on balancing chemical equations: Enter an equation of a chemical reaction and click 'Balance'. The answer will appear below Always use the upper case for the first character in the element name and the lower case for the second character. Examples: Fe, Au, Co, Br, C, O, N, F. Compare: Co - cobalt and CO - carbon monoxide

Balancing Equations Worksheet 1 Answer Key Equations Worksheets
A balanced chemical equation shows the same number of each type of atom on both sides of the arrow. Questions Tips & Thanks Want to join the conversation? Sort by: Top Voted Gabrielle M. 9 years ago I'm working on Chemical Reactions: Double and Single Replacement on FLVS.